Contract Manufacturer Certification
The award for the highest production quality of dental implants
Certification and reward for outstanding efforts of contract manufacturers
The CleanImplant Foundation has long been a widely recognized authority among implantologists for independent evaluations, ratings, and information on the quality and cleanliness of dental implants. Based on the globally established CleanImplant consensus guideline on the cleanliness of dental implants, the independent non-profit organization is also awarding a unique certification to contract manufacturers producing implants for various trade brands.
The certificate not only confirms the highest production quality. Annual audits and unannounced product inspections by accredited testing laboratories also ensure the sustainability of efforts in the production of implants even before the final packaging and sterilization process. Legal manufacturers and implant suppliers benefit from this quality initiative.

Contract manufacturer Certification
Award winners

As a world innovation leader for advanced ceramics, CeramTec can help you to find the right solution and to achieve the desired level of differentiation. We are drawing on more than 40 years of experience in dental applications and specialist know-how in ceramics. With over 3,500 employees, the CeramTec Group is a global leading manufacturer of advanced ceramics for medical applications. CeramTec’s ceramic implants are metal-free and exhibit excellent biocompatibility, chemical stability, and high wear resistance.


Komet Medical as part of the family-run company Gebr. Brasseler GmbH & Co. KG, can look back on many years of experience and has outstanding know-how in the development and production of implant drills and drivers for dental implantology. The range of services offered by Komet Custom Made includes competence in all aspects of ceramics, many years of design expertise, sophisticated manufacturing and packaging know-how relating to ceramic implants as well as abutments and implantology drills. Besides a special production line with high-precision machines and systems specifically designed for ceramic devices, the quality management, fulfillment of regulatory requirements based on the MDR and logistic services such as VMR and VMI are treated with absolute priority.

License Criteria
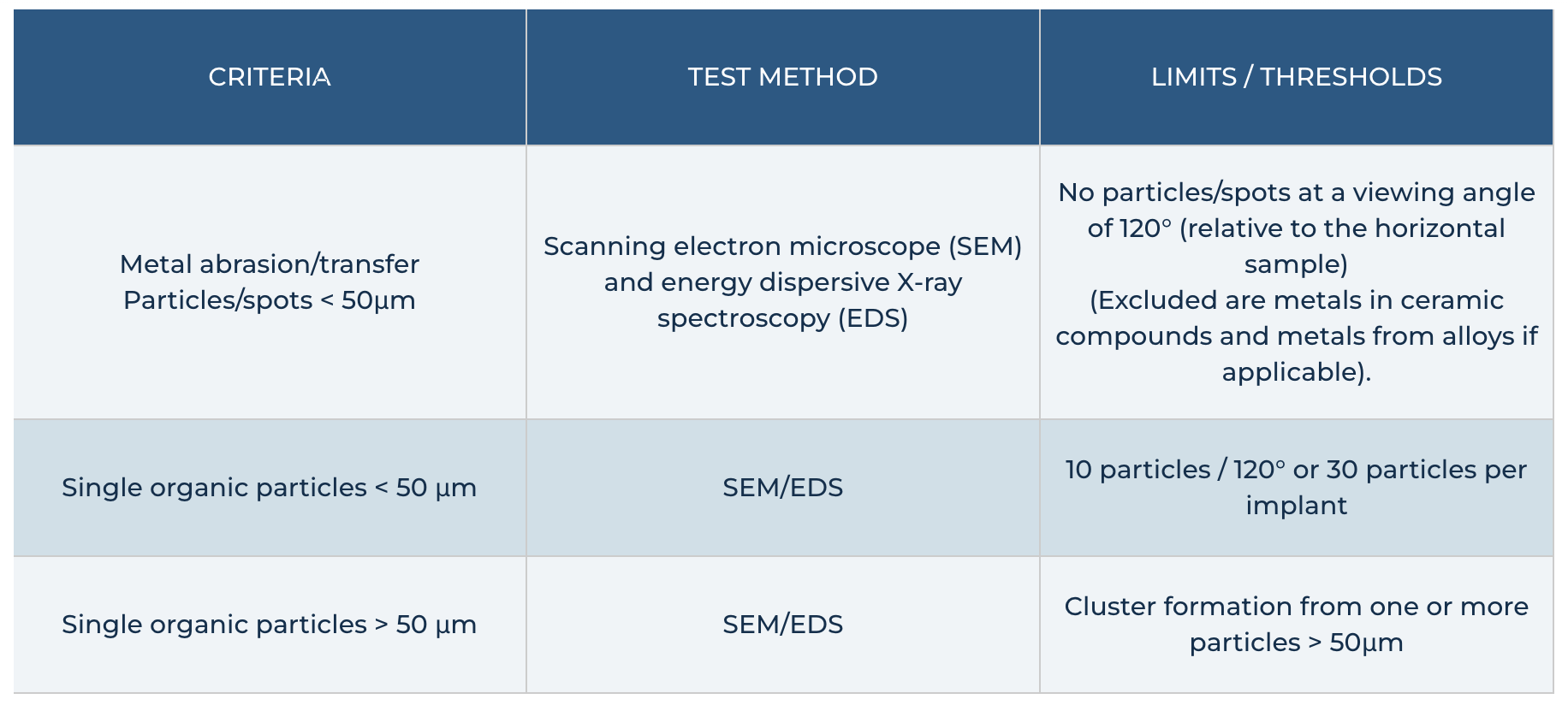
License criteria for the Certified Production Quality Seal relate to the CleanImplant Guideline for the approval of the Trusted Quality Mark. This consensus document was released and published in September 2017.