CleanImplant
Publications

Dental Asia article about the CleanImplant Initiative and its Founder
With great pleasure CleanImplant announces the recent Dental Asia article about the CleanImplant Initiative and its Founder, Dr. Dirk Duddeck. It sheds light on one of the most underestimated factors contributing to peri-implantitis: factory-related contamination of new, sterile-packaged implants — an issue with far-reaching clinical, ethical, and economic consequences.

Contaminants on Titanium and Ceramic
Dental implants are designed to replace the root of the missing tooth and serve as the foundation for the desired restoration. Whether implants are titanium or ceramic (ie, zirconia), they “bond” to the bone through a process called osseointegration in which the bone attaches to the implant surface. Although dental implants have become a mainstream treatment option, the profession is witnessing an increase in the reported incidence of peri-implantitis and accompanying peri-implant bone loss. These reports relate to bone loss at the interface of the implant surface, which is calibrated as bone-to-implant contact. Fortunately, it is commonly accepted that the incidence of peri-implantitis is not actually increasing; the identification of it is.

Does The Implants Condition At Manufacturing And Packaging Have An Effect On Peri-Implantitis?
Dental implants have become standard treatment for replacement of single missing teeth, the fully edentulous arch, and edentulous portions of the arch. Dental implants have maintained a very high level of success for decades. Yet, problems may arise related to the bone to implant contact (BIC) leading to bone loss at the interface (peri- implantitis) and potential loss of the implant.
Peri-implantitis is a pathological process that occurs at the bone surrounding dental implants characterized by inflammation of the soft and hard tissues adjacent to the
implant, which leads to progressive bone loss supporting the implant. When not treated early enough, eventual loss of the implant can result. Peri-implantitis may appear early in the life of one or more implants or many years after restoration of the implant(s) and may progress slowly or in an accelerated manner depending on the causative factor and the patient’s host immune system.

Compendium Peri-implantitis and the Effect of the Implant Surface at Placement Compendium

Enhanced Osteoblast Adhesion and Proliferation on Vacuum Plasma-Treated Implant Surface
In this study, we propose a vacuum plasma device for surface treatment of dental implants. This plasma device was designed to allow direct installation of sealed implant packaging containing the dental implant. In this manner, the dental implant could be treated with plasma under a moderate vacuum environment while remaining in a sterile condition. To assess the osseointegration efficiency, in vitro experiments using sandblasted, large grit, acid etching (SLA), calcium coated-SLA (CaSLA), and calcium coated-SLA with plasma treatment (PCaSLA) were performed. The implant surface was observed with scanning electron microscope (SEM) before and after plasma treatment. Thereafter, protein adsorption, cell adhesion, proliferation, and differentiation efficiency were investigated on the surface of each implant type using saos-2, an osteoblast. Plasma treatment significantly improved protein adsorption, cell adhesion, and cell proliferation efficiency compared to both CaSLA and SLA without damaging the calcium coating. According to the findings, the proposed vacuum plasma device has shown the potential to improve osseointegration efficiency. We believe that this plasma technology can be an innovative chairside solution that can be easily handled in the clinical field with superb usability. Jeon, H.J.; Jung, A.; Kim, H.J.; Seo, J.S.; Kim, J.Y.; Yum, M.S.; Gweon, B.; Lim, Y. Enhanced Osteoblast Adhesion and Proliferation on Vacuum Plasma-Treated Implant Surface. Appl. Sci. 2022, 12, 9884. https://doi.org/10.3390/app12199884

Improvement of osseointegration efficacy of titanium implant through plasma surface treatment
A novel plasma treatment source for generating cylindrical plasma on the surface of titanium dental implants is developed herein. Using the titanium implant as an electrode and the packaging wall as a dielectric barrier, a dielectric barrier discharge (DBD) plasma was generated, allowing the implant to remain sterile. Numerical and experimental investigations were conducted to determine the optimal discharge conditions for eliminating hydrocarbon impurities, which are known to degrade the bioactivity of the implant. XPS measurement confirmed that plasma treatment reduced the amount of carbon impurities on the implant surface by approximately 60%. Additionally, in vitro experiments demonstrated that the surface treatment significantly improved cell adhesion, proliferation, and differentiation. Collectively, we proposed a plasma treatment source for dental implants that successfully removes carbon impurities and facilitate the osseointegration of SLA implants. Lee, H., Jeon, H.J., Jung, A. et al. Improvement of osseointegration efficacy of titanium implant through plasma surface treatment. Biomed. Eng. Lett. 12, 421–432 (2022). https://doi.org/10.1007/s13534-022-00245-9

Interview with Dental Online Channel
Rückstände am Implantat – trotz steriler Verpackung Welche Folgen hat es für Patienten, wenn ein Implantat mit Partikeln aus dem Produktions- und Verpackungsprozess belastet ist? Und welche Implantate sind wirklich rückstandsfrei? Das untersucht die CleanImplant Foundation. Im exklusiven Telefoninterview mit dem Dental Online Channel erläutert der Managing Director Dr. Dirk Duddeck die Hintergründe des Projekts.

Cover Story Article (German)
zm berichtet über das CleanImplant Projekt (CleanImplant project has become a cover story of the official journal of the German Dentists Association) In der aktuellen Ausgabe der zm werden Aufgaben und Ziele des CleanImplant Projekts im Detail erklärt. Falls Sie sich für unsere Arbeit interessieren und Sie mehr dazu erfahren wollen, melden Sie sich für unseren Newsletter (Englisch/Deutsch) hier an. Erfahren Sie alles über aktuelle Untersuchungen und zukünftige Analyseergebnisse zu einzelnen Herstellern. Sie wollen uns Ihren Kommentar senden oder haben konkrete Fragen? Dann schreiben Sie uns auf Deutsch hier. Ihnen gefällt unser Projekt? Dann empfehlen Sie uns bitte im Kollegenkreis weiter. Helfen Sie mit, dass unsere Kampagne erfolgreich wird! Dafür im Voraus herzlichen Dank.

How safe is your implant?
Global initiative for clean dental implants Residues on sterile packaged implants, in particular organic particles from the production or packaging process, are highly suspected of being responsible for an incomplete osseointegration of dental implants or even a loss of bone in the early healing period. Studies from recent years have shown that neither the CE mark nor the FDA clearance can provide a reliable indication of the cleanliness of dental implants. In March 2017, a new initiative was presented at the IDS in Cologne, which is focusing on this topic for the protection of both the users and the patients. Article by Dr. Dirk U. Duddeck published in: implants 2-2017

Impurities on Sterile Packaged Implants:
Is the Rise of Cheap Copy-Cat Products a Danger for our Patients? 30 years after the first SEM research about contaminations on dental implants by Wahl et al., we still can find major impurities on many sterile implants today. Not only clinical1,2 but also legal implications may occur in the future if practitioners are using implants of inferior quality. The quality of a dental implant is always the result of appropriate quality management. Results of SEM/EDS analyses in 2017 give cause for concern. 1) Trindade R, Albrektsson T, Tengvall P, Wennerberg A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin Implant Dent Relat Res 2016; 18: 192-203.
2) Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A., Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res 2014; 16(2): 155–65. doi: 10.1111/cid.12142.

„All that glitters ain´t gold“
Can ceramic implants meet higher expectations? When Prince wrote his song “Gold”, of which the headline of this article quotes the refrain, he tried to explain the problem of having exaggerated expectations in a relationship. Presumably, he was not thinking about an ideal material for dental implants. Zirconium dioxide may have some advantages in comparison with titanium or titanium alloys. Better aesthetic appearance in case of significant bone loss and low plaque affinity are benefits of this material. As the production process differs from titanium implants, one might expect that the surface cleanlinessof zirconia implants further makes a difference. Article by Dr. Dirk U. Duddeck, published in: ceramic implants 2-2018

Interview with Prof. Hugo de Bruyn
We speak about impurities on dental implants and discuss the need for a non-biased, independent and global quality assessment. We are honored and encouraged to welcome Professor de Bruyn as a new member of the CleanImplant Scientific Advisory Board.
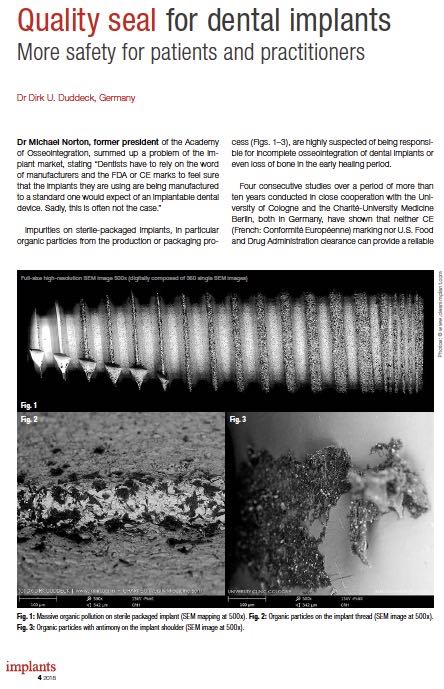
Quality Seal for Dental Implants
Dr Michael Norton, former president of the Academy of Osseointegration, summed up a problem of the implant market, stating “Dentists have to rely on the word of manufacturers and the FDA or CE marks to feel sure that the implants they are using are being manufactured to a standard one would expect of an implantable dental device. Sadly, this is often not the case.” Impurities on sterile-packaged implants, in particular organic particles from the production or packaging process (Figs. 1–3), are highly suspected of being responsible for incomplete osseointegration of dental implants or even loss of bone in the early healing period. Four consecutive studies over a period of more than ten years conducted in close cooperation with the University of Cologne and the Charité-University MedicineBerlin, both in Germany, have shown that neither CE (French: Conformité Européenne) marking nor U.S. Food and Drug Administration clearance can provide a reliable Article by Dr. Dirk U. Duddeck, published in: implants 4-2018

Quality Deficiencies of Sterile Packaged Dental Implants
How Much Safety can FDA Clearance or the Newly Implemented MDR in Europe Guarantee? When dentists began using dental implants in the late 80s, no implant was launched on the market without comprehensive studies and pre-launch tests in renowned dental centers, normally linked to universities of high international prestige. With an increasing number of dental implant manufacturers and suppliers, dental professionals can choose from a variety of implant types with different geometries, surface treatments, and core materials. However, can dentists blindly trust the quality of these implants? In 2019, the FDA released two decades of previously unpublished data containing millions of malfunctions by medical devices, including 2.1 million reports of failed dental implants. This amounts to more than 100,000 reports in 2018 alone – most of them related to a lack of osseointegration. Opinion article by Dr. Joao Pimenta, Dr. Dirk Duddeck, published in: JDODT 4-2020

Will any dental Implant do?
Opinion piece by Dr. Michael R. Norton Implant surfaces can be contaminated with organic and metallic impurities, which might impact upon quality of osseointegration and risks for peri-implantitis. It is neither appropriate nor possible to extrapolate data from one implant brand to another. Due to the exponential growth in the number of implant brands and types available, there is an increasing need for a central UK dental implant registry.
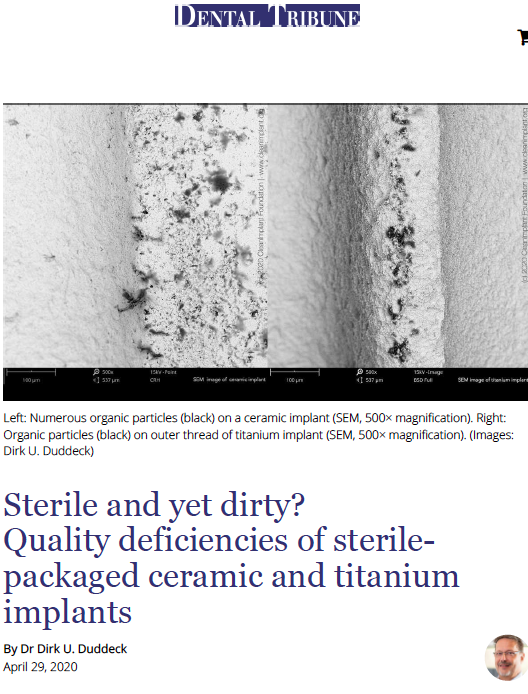
Sterile but yet dirty?
In 2019, the FDA released two decades of previously unpublished data and 2.1 million reports of failed dental implants from which more than 100,000 reports referred to 2018 alone. Most of these failures related to a lack of osseointegration, raising major concerns among dentists in the US and abroad as the number of additional unreported losses is likely to be much higher. Comments made by manufacturers, regarding these figures, focus on patients with unfavorable clinical preconditions and even blame dentists for their lack of experience and training. Is this the whole truth? In a recent study, conducted by the non-profit CleanImplant Foundation in collaboration with the Charitè University Berlin, more than 100 different sterile-packaged implants – including ceramic and titanium implants – from 80 implant brands were analyzed. SEM imaging and elemental analysis (EDS) were performed in an officially accredited testing laboratory, according to DIN EN ISO/IEC 17025. Almost every second implant sample that was unpacked under cleanroom conditions and analyzed in the SEM showed considerable contamination, i.e. unwanted particles originating from the manufacturing, handling or packaging of the implant. These contaminants on sterile packaged implants, especially organic particles from the manufacturing or packaging process, can cause an uncontrolled foreign body reaction resulting in osteoclastogenesis, leaving rough areas of the implant surface exposed to bacterial colonization. Article by Dr. Dirk U. Duddeck, published in: Dental Tribune U.S. 4-2020

About the threshold in quality deficiencies of sterile-packaged dental implants
Interview in German language with Dr. Dirk U. Duddeck, published in PIP Journal Germany 9-2020

On the Cleanliness of Different Oral Implant Systems – A Pilot Study
Journal of Clinical Medicine Abstract: (1) Background: This paper aimed to compare the cleanliness of clinically well-documented implant systems with implants providing very similar designs. The hypothesis was that three well-established implant systems from Dentsply Implants, Straumann, and Nobel Biocare were not only produced with a higher level of surface cleanliness but also provided significantly more comprehensive published clinical documentation than their correspondent look-alike implants from Cumdente, Bioconcept, and Biodenta, which show similar geometry and surface structure. (2) Methods: Implants were analyzed using SEM imaging and energy-dispersive X-ray spectroscopy to determine the elemental composition of potential impurities. A search for clinical trials was carried out in the PubMed database and by reaching out to the corresponding manufacturer. (3) Results: In comparison to their corresponding look-alikes, all implants of the original manufacturers showed—within the scope of this analysis—a surface free of foreign materials and reliable clinical documentation, while the SEM analysis revealed significant impurities on all look-alike implants such as organic residues and unintended metal particles of iron or aluminum. Other than case reports, the look-alike implant manufacturers provided no reports of clinical documentation. (4) Conclusions: In contrast to the original implants of market-leading manufacturers, the analyzed look-alike implants showed significant impurities, underlining the need for periodic reviews of sterile packaged medical devices and their clinical documentation. Dirk U. Duddeck 1,2, *, Tomas Albrektsson 3 , Ann Wennerberg 4 , Christel Larsson 5 and Florian Beuer

Publication: Quality assessment of five Ceramic Implant Systems
Three Zirconia Implants revealed significant contaminants In October 2021, a scientific paper analyzing a total of 25 implant samples from five manufacturers was published in the International Journal of Oral & Maxillofacial Implants. The study was initiated by Dirk Duddeck, CleanImplant’s head of research, and conducted with renowned scientists such as Tomas Albrektsson, Ann Wennerberg, Florian Beuer, Christel Larsson and Jaafar Mouhyi. The results of this study showed that, in principle, relatively clean implant surfaces are technically possible with ceramic implants, which should, of course, be the aim for all implant materials. The surfaces of the Champions implants (ZV3) and the Z-Systems implants were relatively clean. In contrast, the other investigated surfaces of the vitaclinical, TAV Dental, and ZiBone implants revealed significant contamination on their surfaces.