Sterile but yet dirty?
Quality deficiencies of sterile-packaged ceramic and titanium implants
In 2019, the FDA released two decades of previously unpublished data and 2.1 million reports of failed dental implants from which more than 100,000 reports referred to 2018 alone. Most of these failures related to a lack of osseointegration, raising major concerns among dentists in the US and abroad as the number of additional unreported losses is likely to be much higher. Comments made by manufacturers, regarding these figures, focus on patients with unfavorable clinical preconditions and even blame dentists for their lack of experience and training.
Is this the whole truth?
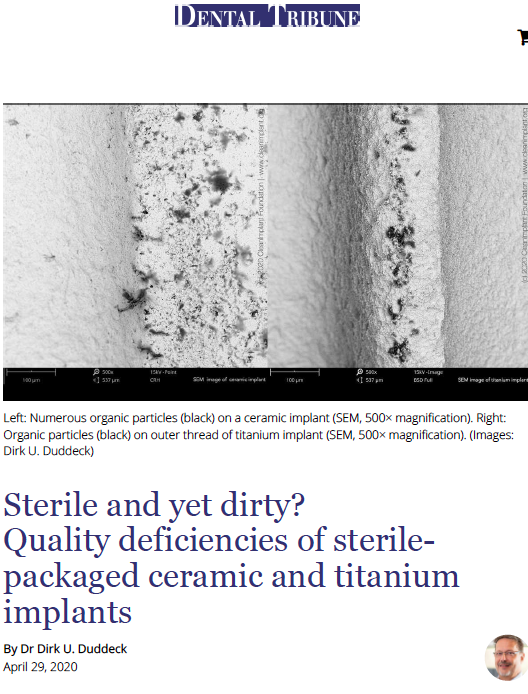
In a recent study, conducted by the non-profit CleanImplant Foundation in collaboration with the Charitè University Berlin, more than 100 different sterile-packaged implants – including ceramic and titanium implants – from 80 implant brands were analyzed. SEM imaging and elemental analysis (EDS) were performed in an officially accredited testing laboratory, according to DIN EN ISO/IEC 17025. Almost every second implant sample that was unpacked under cleanroom conditions and analyzed in the SEM showed considerable contamination, i.e. unwanted particles originating from the manufacturing, handling or packaging of the implant. These contaminants on sterile packaged implants, especially organic particles from the manufacturing or packaging process, can cause an uncontrolled foreign body reaction resulting in osteoclastogenesis, leaving rough areas of the implant surface exposed to bacterial colonization.
Article by Dr. Dirk U. Duddeck, published in: Dental Tribune U.S. 4-2020