Approved by
Clean Implant

This “Approved by CleanImplant Seal” was established to provide guidance on the outstanding properties of a medical device, digital technology, software, implant care product, or a technology that has been proven in accredited testing laboratories or in a comparable manner to support successful clinical outcomes in the field of dental implantology.

Medical Devices
Surgical Guides & Bone Augmentation
Digital Technologies
Implant Stability Measurement Devices
Guided Implant Treatment Planning software
Implant Care Products

Medical Devices

Digital Technologies

Guided Implant Treatment Planning software

Surgical Guides & Bone Augmentation

Implant Stability Measurement Devices

Implant Care Products
Approved therapy support
Award winners






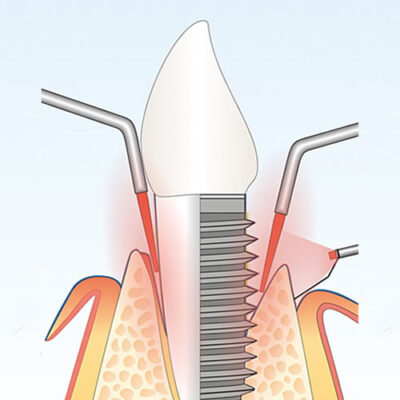
HELBO Laser Therapy System
Advanced Antimicrobial Photodynamic Therapy (aPDT)
HELBO Laser Therapy utilizes antimicrobial photodynamic therapy (aPDT) to target and eliminate harmful bacteria, promoting faster healing without the use of antibiotics.


PRGF – ENDORET® is a plasma rich in platelets (PRP) technology for obtaining autologous proteins from the patient’s own blood.
Using a concrete protocol and a system of specific components, PRGF – ENDORET® provides for the elaboration of up to 4 therapeutic formulations that can be used in several clinical applications.

Apply now for the
Approved by CleanImplant
Certification
if you supply a medical device, digital technology, software, implant care product, or a technology that has been proven to support successful clinical outcomes in the field of dental implantology.