Trusted Quality Seal
The independent Quality Assessment of Dental Implants
The Award for
Outstanding Quality
The CleanImplant Foundation global quality label, the TRUSTED QUALITY seal is the result of a peer-review process after a thorough analysis of randomly selected implant samples (see graphic below). The seal is an expression of the highest quality control in the manufacture of dental implants and ensures an impeachable ethical standard for our patients. The project’s mission is to raise awareness of the qualitative difference among manufacturers. The CleanImplant TRUSTED QUALITY seal enables manufacturers to increase their market standing. The label differentiates one company from all others. Customer loyalty and satisfaction are significantly enhanced. Marketing, sales and public relations departments can use the quality seal and SEM images to communicate verifiable evidence and persuasive arguments to demonstrate transparency in their production standards and a moral and ethical commitment to excellence.

Strengthen your ethical corporate culture and brand image by integrating the CleanImplant support in your individual marketing strategy and make use of the worldwide impact the global quality campaign provides.
Be an integrated part of worldwide conference lectures/presentations about the CleanImplant Project where you will be mentioned as one of the campaign-supporting partners.
Lecturing at your company events: We present your new Quality Seal and make you a part of research projects initiated by the CleanImplant Foundation.
CleanImplant Trusted Quality Seal
Five-Step Approach
Step 1
Neutral sampling of 5 implants
Batch-spanning random sampling: Three implants are ordered ex-factory, and two implants of the same type are purchased via mystery shopping from practices.


Step 2
Unpacking and scanning under clean room conditions
All five collected samples are carefully unboxed, mounted and scanned in a clean room environment according to Class 100 FED- STD-209E and Class 5 DIN EN ISO 14644-1.
Step 3
Externally audited process of analysis
Scanning electron microscope (SEM) imaging and elemental analysis (energy-dispersive X-ray spectroscopy) are performed according to the DIN EN ISO/IEC 17025 accreditation process (competence of testing and calibration laboratories). The independent test laboratories are regularly monitored in external audits by the German accreditation body DAkkS.


Step 4
Full-size and high- resolution SEM images
A special full-size, high-resolution SEM image—digitally composed of more than 360 single SEM images at 500× magnification— always shows the implant surface from shoulder to apex.
Step 5
Peer-review process
Two members of the scientific advisory board independently review the comprehensive analysis report and sufficient clinical documentation or multi-annual Post-Market Clinical Follow-Up studies of the analysed implant system showing survival rates of more than 95% for the device or device family.

The source of information for more than 35.000 dental professionals. Every year.
With a continually expanding Internet community, dentists from more than 140 countries, find detailed information and data of analysis to provide knowledgeable-based decision-making for choosing implant systems.

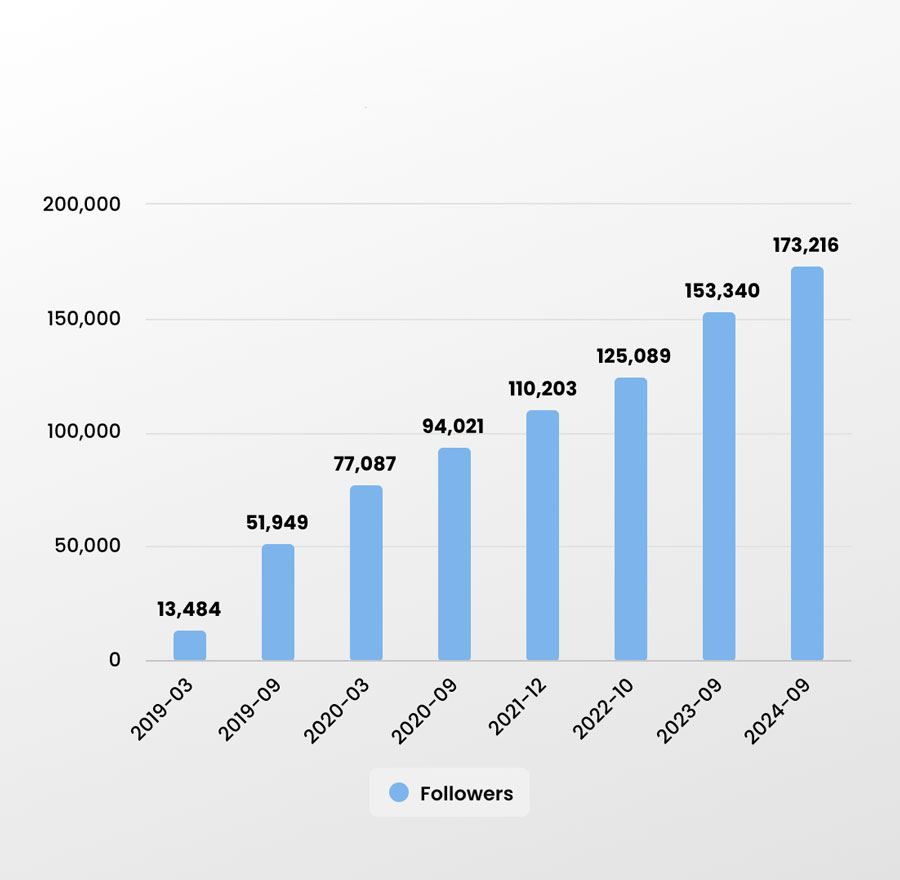
Dynamic growth of the CleanImplant community
Since the CleanImplant Facebook page was published in June 2018, the community has grown to more than 170,000 subscriptions – the result of a constant stream of professional information and implant-related posts.
Worldwide Expertise
To date, 50 international key opinion leaders in the field of implant dentistry share our mission and values.
Representing 20 countries, these Ambassadors support the network and spread the information world-wide by using images and results of recent CleanImplant research projects in international lectures and scientific publications.

Are you interested in the Trusted Quality Seal for your implant system(s)?
Start your application here