Research
Scientific background
Quality Assessment Studies & Basic Research
FDA requires additional test procedure long used by CleanImplant
FDA recommends additional test procedure long used by CleanImplant
On October 15, 2024, the US Food and Drug Administration (FDA) published a new guidance document for the simplified approval of dental implants. In addition to the well-known toxicological tests (ISO 10993-5), this guidance document recommends a “surface cleanliness analysis” for the first time and thus focuses on the possible immunological effects of particulate contamination.
We are grateful that the FDA recognizes the comprehensive testing procedure developed by our group back in 2016 for a long series of quality assessment studies.

Highest Standards for the Implant Analysis
All collected samples are subjected to the same quality assessment protocol. Imaging and elemental analysis in the scanning electron microscope are performed by independent laboratories that comply with the highest standards of scientific research. These laboratories are officially accredited according to ILAC / MRA – DIN EN ISO/IEC 17025:2018 (general requirements for the competence of testing and calibration laboratories). This includes the quality standard according to:
- DIN EN ISO 9001:2015 and the implementation of international standards for microbeam analysis scanning electron microscopy, such as
- ISO 16700:2016 (Guidelines for calibrating image magnification)
- ISO 14595:2014 (Guidelines for the specification of certified reference materials)
- ISO 22309:2015 (Quantitative analysis using energy-dispersive spectrometry EDS).


ISO Class 5 Cleanroom Environment DIN EN ISO 14644-1
The unpacking of the implants themselves, the fixation of the samples on a specimen holder, and even the imaging process and elemental analysis by the scanning electron microscope take place in a particle-free environment that meets class 100 cleanroom requirements according to United States Federal Standard (US FS) 209 and ISO class 5 according to DIN EN ISO 14644-1.
Scanning Electron Microscopes
Scientific workstations in the testing laboratories are, for example, Scanning Electron Microscopes provided by Thermo Fisher Scientific, equipped with a high-sensitivity backscattered electron detector that allows compositional and topographical imaging modes. Energy Dispersive X-ray Spectroscopy (EDS) analysis is performed with a thermoelectrically cooled Silicon Drift Detector (SDD). Active area: 25mm2; Energy resolution Mn Kα ≤ 140 eV; Processing capabilities: Multi-channel analyzer with 2048 channels at 10 eV/ch; Max. input count rate: 300,000 cps.

High-resolution SEM Mapping Images
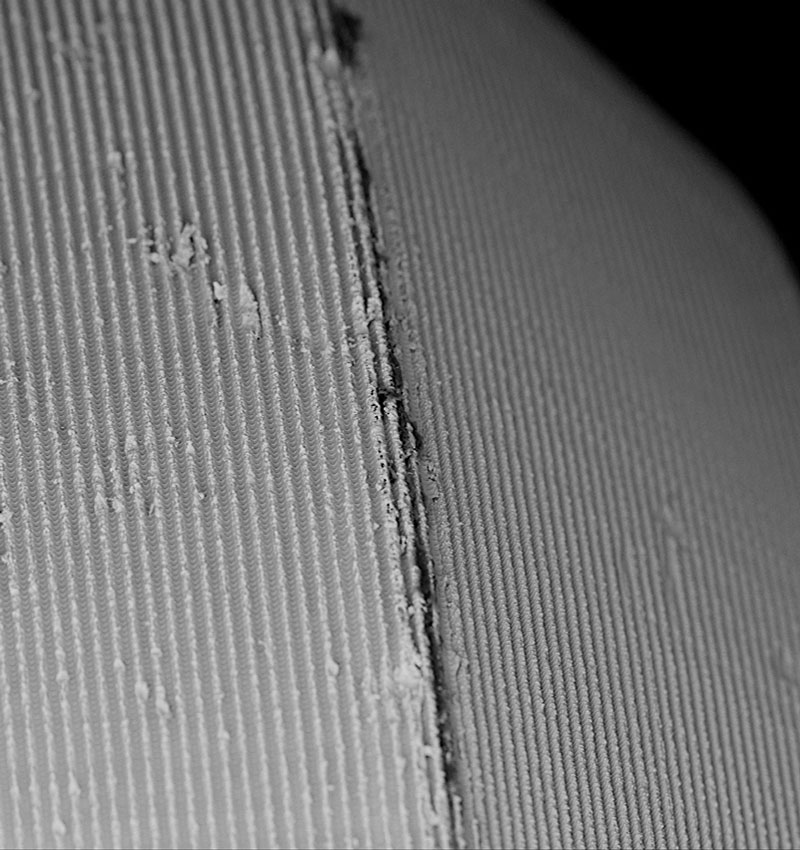
Implants are scanned at a magnification of 500x from shoulder to apex in the “Image-Mapping” mode. This technique produces up to 600 single high-resolution SEM images that are digitally composed to one large image with an extremely high resolution, the FSHR image (Full-Size High-Resolution). Mapping images of implant samples in this study show the complete surface at an angle of view of 120°. Thus, the detailed report of every implant provides information not only on single sections or small areas of the sample but always a complete and precise overview of the implant surface. The composed FSHR mapping image allows us to count particles in the visible field and identify areas of interest for subsequent spot analyses.
Detailed SEM-Imaging and Analysis into the Sub-micron Range
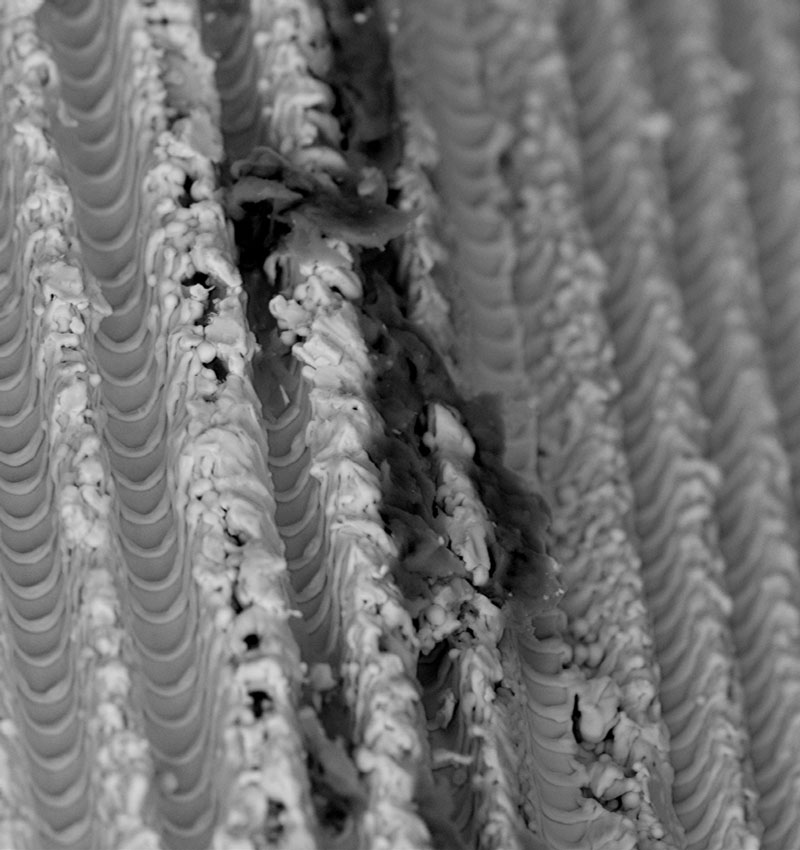
Scanning electron microscopy (SEM) enables the topical evaluation of the implant surface. The high-sensitivity backscattered electron (BSE) detector produces material-contrast images of implants made of titanium, titanium alloy, and zirconia up to a magnification of 5,000x, showing particles as small as 0,5 µm in diameter. BSE imaging provides additional information about the implant sample, such as the chemical nature and allocation of different remnants or superficial contaminants.
Time-of-Flight Secondary Ion Mass Spectroscopy
(ToF-SIMS)
This specific method of analysis, performed at the Tascon Laboratory in Muenster, Germany, offers detailed information about the chemical composition of impurities that have been detected in the preceding scanning electron microscopy. The method provides information on the atomic and molecular structure of the upper-most monolayers of a substrate on an analysis area of 500 × 500 µm with sensitivity in the parts per million range and a lateral resolution of up to 100 nm. Comparison of the spectra with known chemical substances allows precise material determination of any implant contaminations.
Pesticides and Aggressive Surfactants
The unique combination of SEM imaging, Energy Dispersive X-ray Spectroscopy, and Time-of-Flight Secondary Ion Mass Spectrometry could already identify plastics such as polyoxymethylene and reveal even serious contaminations on dental implants such as dodecyl benzenesulfonic acid (DBSA), or didecyldimethylammonium chloride (DDAC-C10). Notably, DBSA is an aggressive surfactant classified as a „hazardous substance“ by the EPA. The pesticide DDAC-C10 causes the disruption of intermolecular interactions and dissociation of lipid bilayers. Neither DDAC nor the cell-toxic and surface-active chemical DBSA should be found – not even in residual quantities – on sterile-packaged implants intended for use in patients.
source: Tascon GmbH, Münster Germany
Publication of CleanImplant’s Quality Assessments
Our research projects lead to publications in renowned scientific journals. We share our data of analysis with dentists worldwide for the well-being of all patients.

Interested in participating?
Please fill out the form below if you want to participate in the Implant Study 2024-2025
Implant Study 2024/25
Global Quality Assessment of Dental Implants by SEM+EDS